On 4th Oct, 2017, European Commission submitted notifications of G/TBT/N/EU/519 and G/TBT/N/EU/520, which meant to add Trimellitic anhydride (TMA) and dicyclohexyl phthalates (DCHP) into the list of the Substances of Very High Concern (SVHC).
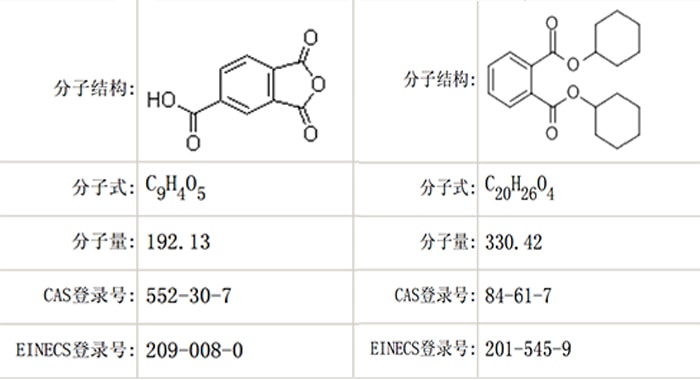
Trimellitic anhydride(TMA), EC number 209-008-0 and CAS number 552-30-7. Scientific evidence suggests that TMA is a respiratory sensitizing substance of probable serious effects on human health, which should get a widely concern. In August 2016, Netherlands submitted to the European Chemicals Agency a dossier which proposes adding TMA into the SVHC. In December 2016, the Member State Committee of the Agency (MSC) considered that TMA meets the conditions for identification as a substance of very high concern, in accordance with Article 57(f) of Regulation (EC) No 1907/2006. On 17th January 2017, the above opinion was referred to the Committee and gained its approval.
Dicyclohexyl phthalates(DCHP), EC number 201-545-9 and CAS number84-61-7. According tothe Regulation (EC) 1272/2008 regarding classification, label, and packaging, DCHP is classified as toxic for reproduction category 1B, and its endocrine disrupting properties can cause serious effect on human health and environment. In February 2016, Switzerland submitted in ECHA a dossier which proposes adding DCHP into the SVHC. In June 2016, the above opinion was referred to the Committee and gained its approval. The commission notes the unanimous agreement in the MSC for the toxic for reproduction properties, but for the endocrine disrupting properties they didn’t reach an agreement. European Committee finally confirmed that DCHP shall be added into SVHC, for its 1B reproduction toxicity and endocrine disrupting properties by obtaining the opinion MSC from ECHA.
C&K Testing reminds enterprises that the list of the Substances of Very High Concern(SVHC) update biannually, so far there’re 174 substances in the SVHC.
Click here for more information about SVHC or download the latest SVHC list.
