The German Federal Institute for Risk Assessment (hereinafter referred to as BfR) is an agency directly under the federal government, which is mainly responsible for identifying and assessing safety risks in food, chemicals, and consumer products (including food contact materials, cosmetics, toys, textiles, and tobacco, etc.). And provide risk management decision-makers with scientific advice on avoiding and reducing risks (including formulating risk limit recommendations). The recommendations on food contact materials formulated by BfR are one of them. Although these recommendations are not legislative documents, they are based on EU and German legislation and represent the current level of science and technology. Not only Germany, but also an important basis for learning and reference for EU member states to ensure product compliance.
On July 1st this year, BfR updated its Food Contact Material Rubber Recommendations again. The following is a summary of this update.
Framework Revision
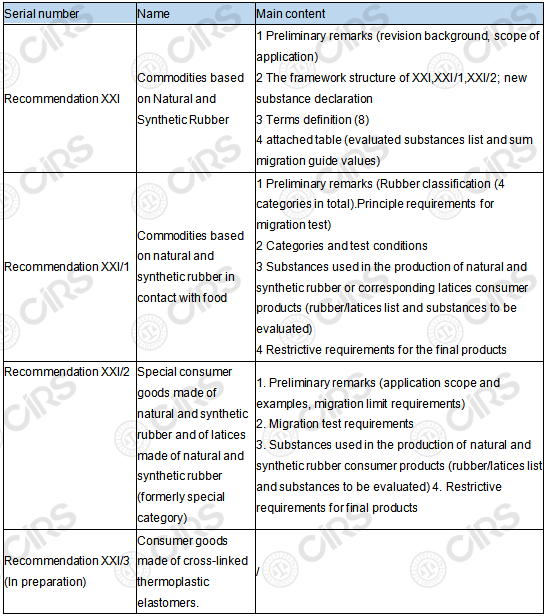
The XXI chapter "Rubber Products" was revised into 3 chapters (XXI, XXI/1, XXI/2) and 1 proposed chapter (XXI/3), replacing the version on July 1st, 2016. The main content of each chapter after the update is shown in the table below.
Table 1 Summary of the update of Recommendation XX1
Technical content revision
The revisions of technical contents are mainly in the scope of application, term definitions, raw and auxiliary materials management, final product requirements, migration test principles and conditions, etc. The specific content changes are as follows:
1. Scope of application and definition of terms
This revision further clarifies the scope of application of the Recommendation, that is, this Recommendation is applicable to consumer products made of natural and synthetic rubber and its latices. For silicone rubber and thermoplastic elastomers (TPE) produced from the monomers and additives listed in the Plastics Regulation (EU) No 10/2011 are respectively controlled by BfR XV "Silicones" and (EU) No 10/2011 regulations.
In order to clarify the meaning of the main components of rubber, the following new term definitions have been added in this revision: definitions of terms for elastomers, rubbers, latices, fillers, plasticizers, anti-aging agents, processing aids and cross-linking agents, which are convenient for enterprises to identify and allow The type of raw and auxiliary materials used.
2. Raw and auxiliary materials requirements
This revision divides the raw and auxiliary materials for rubber into two categories: evaluated and not conclusively evaluated , as shown in the following table.
Table 2 List of raw and auxiliary materials in the Recommendation
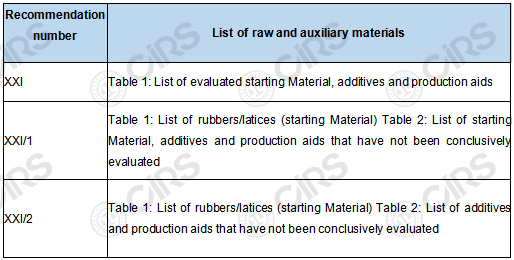
Selection of raw and auxiliary materials: It is necessary to select the corresponding substances in the list for production according to the product categories in Article 3 "Substances for the production of natural and synthetic rubber consumer products" in XXI/1 and XXI/2 respectively. For example, to produce the polybutadiene rubber (BR) listed in XXI/1 Table 1, the materials listed in XXI Table 1 and XXI/1 Table 2 should be selected as the starting materials (monomers).
Restrictive requirements for raw and auxiliary materials: XXI has added migration limits for some substances in the evaluated list (including SML and SML(T)), and stipulated maximum usage for substances in the XXI/1 and XXI/2 that have not been conclusively evaluated. Except for special regulations in the XXI list, other substances use 60 mg/kg as their migration limit.
New substance approval: within two years from the date of issuance, the substances listed in the XXI/1 and XXI/2 conclusively evaluated list can be transferred to the XXI evaluated substance list by submitting an application to BfR.
3. Control of the final product
Compared with the previous versions of Recommendations XXI/1 and XXI/2, the main changes to the final product are as follows:
A new test indicator for migration of aluminum has been added, with a limit of 1 mg/kg.
The limit requirements for overall migration, lead residue, zinc residue, and migration of primary aromatic amines have been revised.
Overall Migration: Unify the overall migration limit of various simulants as 10 mg/dm2 (category 1-3), 60mg/kg (special category);
Lead and zinc: change the residual amount to the migration amount, respectively, the migration of lead shall not be detectable, the detection limit is 0.01mg/kg; the migration limit of zinc is 25mg/kg.
The total migration limit of primary aromatic amines: modified to 0.01mg/kg (except for the accessories of the milking device in Category 3), and the migration limit of PAAs classified as 1A/1B is not detectable, and the detection limit is 0.002 mg /L.
The test indicators such as organic ingredients and zinc dibenzyl dithiocarbamate have been deleted.
4. Migration test principles and test conditions
In terms of migration testing, Recommendation XXI/1 mainly adds the principle requirements for migration testing:
Simulants: The simulants must represent the substance transfers to food that are to be expected
in the worst case. The following can be used:
deionised water
- 10 % ethanol by volume
- 20 % ethanol by volume
- 3 % acetic acid by weight
- 50 % ethanol by volume
- Poly(2,6-diphenyl-p-phenylene oxide), particle size 60-80 mesh, pore size 200 nm
- any vegetable oil with less than 1 % unsaponifiable matter.
The selection of simulants other than water shall be made in accordance with Regulation (EU) No 10/2011.
Ratio of contact area (S) to volume (V) of food or simulant (S/V): When the actual S/V cannot be estimated, the S/V can be assumed to be 5dm2/L (1dm2/200mL).
If the material or article is intended to come into repeated contact with foodstuffs, the migration tests shall be carried out three times on the same sample, each time using a different portion of the food (simulant). Conformity shall be checked on the basis of the migration value found in the third test. If there is conclusive evidence that the migration value does not increase in the second
and third tests and the migration benchmarks are not exceeded in the first test, no further testing is required. If not detectable (ND) is specified for the specific migration benchmark of substance(detection limit is 0.01 mg/kg, except for substances specified otherwise) should be judged for compliance based on the first result.
Migration values for caps, gaskets, stoppers and similar closures are expressed in:
a) mg/kg of food using the actual contents of the container for which the closure is intended, provided that the intended use of the article is known,
b) mg/item, if the intended use of the item is not known.
Recommendation XXI/2 specifies specific migration test conditions according to product category and test items, including simulant, selection of test time and temperature, immersion ratio of product and simulant, and number of tests.
[Original link]
About us
C&K Testing is a leading testing company to render you specialised solutions concerning green and sustainable development of products. Established in 2008, we've helped thousands of customers to minimise the risks of their products to human health and the environment through our testing services.
Combining widely global recognition and extensive local experience, staffed by knowledgeable experts, C&K Testing will help you to gain a competitive advantage in the global marketplace by ensuring product safety and quality, removing trade barriers and optimising manufacturing procedures.
If you have any needs or questions, please contact us at test@cirs-group.com.
