22 February 2018, The EU has notified the WTO of amending Regulation (EU) 10/2011 on plastic FCMs. This amendment authorizes two new substances, extends the authorized use of a previously authorized substance, and decreases the specific migration limits for a previously authorized substance on the basis of new evidence, for materials intended to be in contact with foods. Final date for comments is 23 April, 2018.
The amendments are as follows:
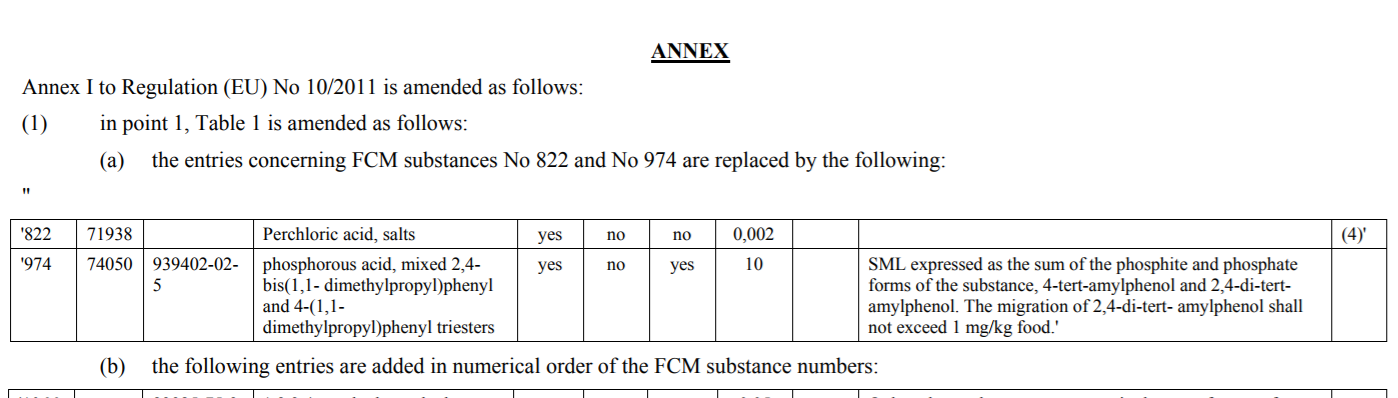
Substance CAS No. Content Amendments perchlorate - the specific migration limit (SML) is
lowered from 0.05mg/kg to 0.002mg/kg. phosphorous acid, mixed 2,4-bis(1,1-dimethylpropyl)phenyl
and 4-(1,1- dimethylpropyl)phenyl triesters 939402-02- 5 the SML increases from 5 to 10mg/kg on the basis of new scientific evidence that this substance is not of a safety concern for the consumer. New Authorisation 1,2,3,4-tetrahydronaphtalene- 2,6-dicarboxylic acid, dimethyl Ester 23985-75-3 the migration of the sum of the substance
and its dimers (cyclic and open chain) should not exceed 0.05mg/kg. [3-(2,3- epoxypropoxy)propyl]trimethoxy silane 2530-83-8 In treated glass fibres, residues of the
substance must not be detectable at 0.01 mg/kg for the substance and 0.06
mg/kg for each of the reaction products (hydrolysed monomers and epoxy-containing
cyclic dimer, trimer and tetramer).
Plastic materials and articles complying with the Regulation before the amendment may be placed on the market during the next 12 months and can be sold until exhaustion of stocks.
This Regulation enters into force 20 days following publication in the Official Journal of the EU.
C&K Testing reminds enterpises to pay attention to the amendment of the FCMs Regulation to prevent the subsequent risk.
|Further Information:
|Our Service:
