The European Commission discussed the outcome and follow-up of the second REACH review on the meeting of 11 June, and proposed restrictions before the sunset date for substances listed on Annex XIV – the authorization list.
ECHA proposed a restriction on four phthalates, DEHP, DBP, DIBP and BBP, which was supported by the Committees for Risk Assessment and Socio-economic Analysis (Rac and Seac) and is currently being discussed in the REACH Committee.
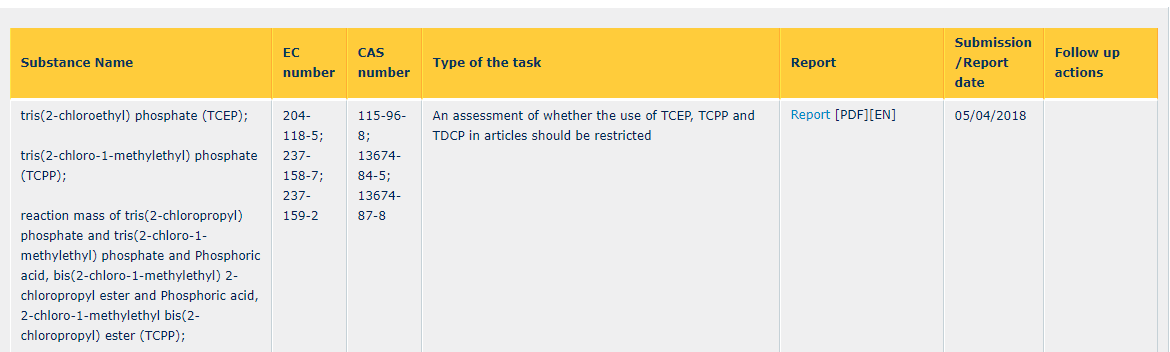
At the same time, ECHA are preparing a restriction for TCEP and lead chromates. For other substances, ECHA will carry out screening reports to assess if a restriction is required over the next six to 12 months.
The second REACH review concludes that REACH has significantly improved the protection of human health and the environment, promoted alternatives to animal testing, and ensured the free movement of chemicals on the EU market.
C&K Testing reminds enterprises to pay attention to the related information of REACH regulation to efficiently prevent the risk.
|Further Information:
REACH Annex XV restriction table
|Our Service:
