9 September 2017, members of the REACH committee approved the amendments to the proposal of CMR at their meeting.
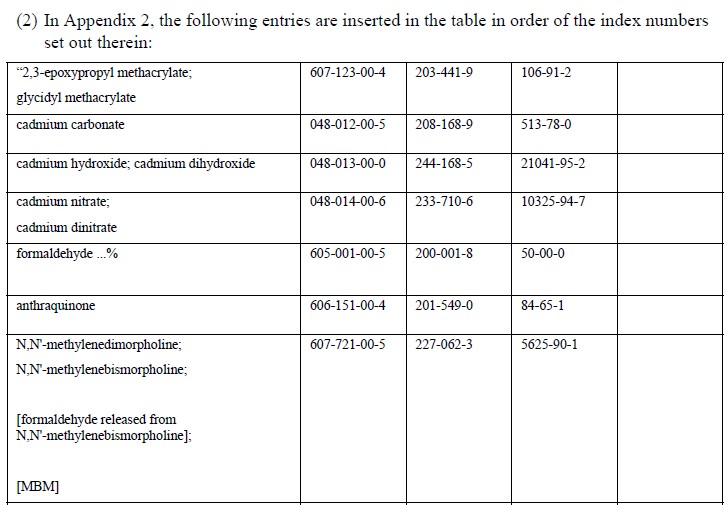
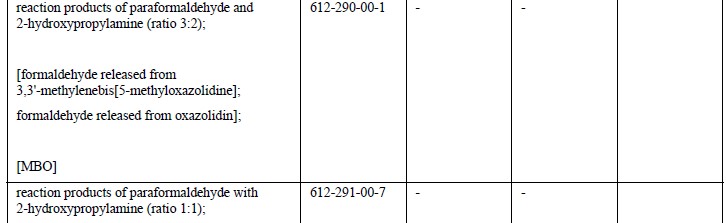
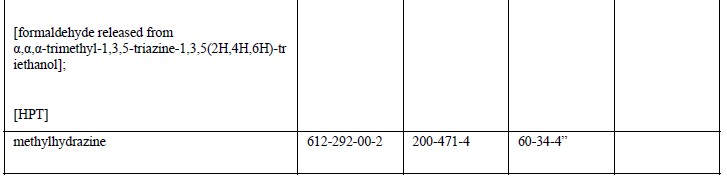
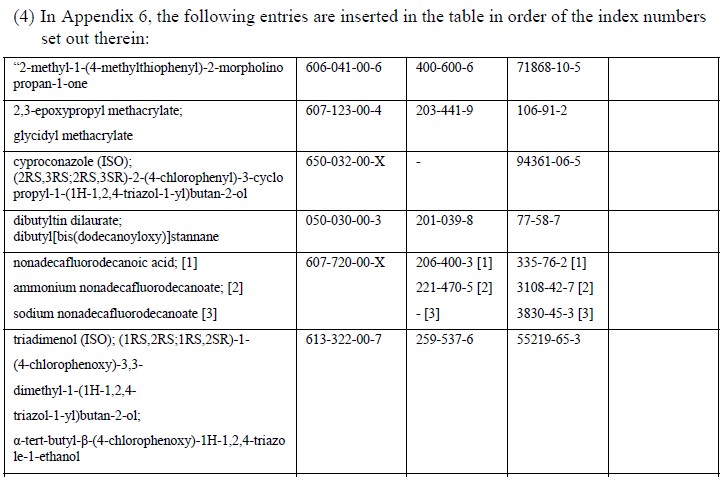
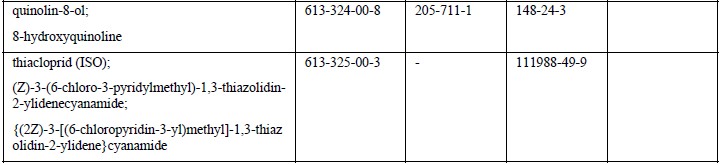
EU member state officials have backed the addition of 18 carcinogenic, mutagenic or reprotoxic (CMR) substances to REACH Annex XVII – the restricted substances list, which have already added 25 CMR substances in April.

Click here to download the Draft Annex to the Regulation
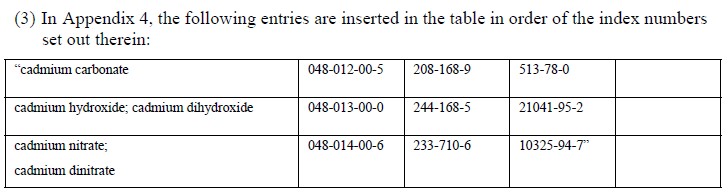
The additions Cadmium nitrate, Cadmium hydroxide and Cadmium carbonate are all identified as new SVHC in a current ECHA consultation, which ended in 20 October 2017.
Based on human and animal evidence respectively, those 18 substances are classified into CMR category 1A or 1B. The marketing or use for supply to the general public of the substances, as well as in mixtures containing them, will be prohibited above specified concentrations.
The Regulation will apply from 1 December 2018 with the exception of formaldehyde, which will apply from February 2018.
|Further information:
ECHA Public Consultation Nine SVHC
Annex XVII to REACH Add CMR Substances
|Our Services:
