23 May 2019, the Official Journal of European Union released the directive (EU) 2019/831, amending the Annex II, III and V to Regulation (EC) No 1223/2009 on cosmetic products. The directive shall enter into force on 11 June 2019. This Regulation shall be binding in its entirety and directly applicable in all Member States.
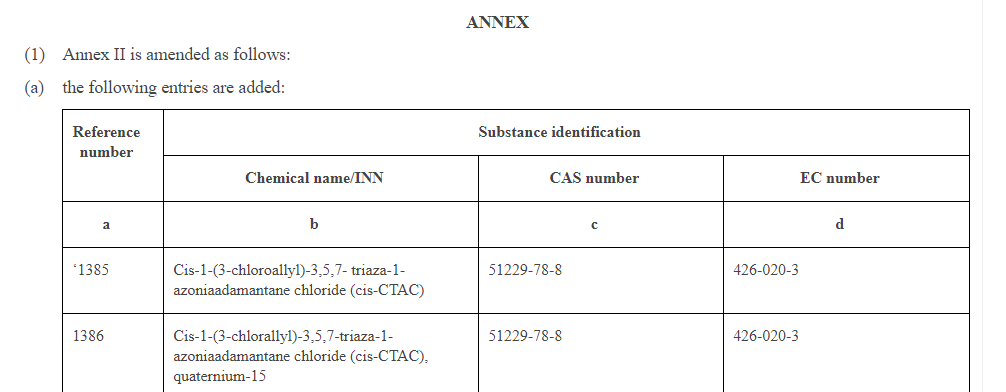
226 entries are added to Annex II and the entry 395 is replaced as follows:
Reference number | Substance identification | ||
Chemical name/INN | CAS number | EC number | |
395 | Hydroxy-8-quinoline and its sulphate | 148-24-2 134-31-6 | 205-711-1 205-137-1 |
Entries 1a, 1b, 7, 13 and 51 to Annex III are deleted, and entries 311 and 312 are added as follows:
Ref No. | Substance identification | Restrictions | Wording of conditions of use and warnings | |||||
Chemical name/ INN | Name of Common Ingredients Glossary | CAS number | EC number | Product type, body parts | Maximum concentration in ready for use preparation | Other | ||
a | b | c | d | e | f | g | h | i |
311 | Diphenyl(2,4,6-trimethylbenzoyl)-phosphine oxide | Trimethyl-benzoyl diphenylphos-phine oxide | 75980-60-8 | 278-355-8 | Artificial nail systems | 5.0 % | Professional use | For professional use only Avoid skin contact Read directions for use carefully |
312 | 2-Furaldehyde | Furfural | 98-01-1 | 202-627-7 | 0.001 % | |||
Reference number | Substance Identification | Conditions | |||||
Chemical name/INN | Name of Common Ingredients Glossary | CAS number | Product type, Body parts | Maximum concentration in ready for use preparation | Other | ||
a | b | c | e | f | g | h | i |
28 | Polyhexa-methylene biguanide hydrochloride | Polyamino-propyl biguanide | 608-723-9 608-042-7 | 0.1 % | Not to be used in applications that may lead to exposure of the end-user's lungs by inhalation | ||
OJ Released the Directive (EU) 2019/831 to Amend the Regulation on Cosmetic Products
|Our Service:
