19 January 2018, the Official Journal of EU issues the EU 2018/79, amending Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food. The new regulation add 2,4,4’-trifluorobenzophenone, 2,3,3,4,4,5,5-heptafluoro-1-pentene, tungsten oxide and the mixture of methyl-branched and linear C14-C18 alkanamides, derived from fatty acids, and also expands the use of scope of (butadiene, styrene, methyl methacrylate, butyl acrylate) copolymer cross-linked with divinylbenzene or 1,3-butanediol dimethacrylate.
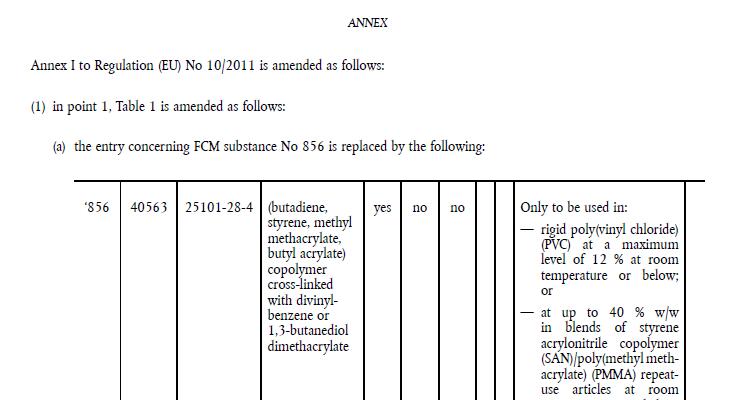
According to EFSA, (butadiene, styrene, methyl methacrylate, butyl acrylate) copolymer cross-linked with divinylbenzene or 1,3-butanediol dimethacrylate (FCM substance No 856), if used as a polymeric additive at up to 40 % w/w in blends of styrene acrylonitrile copolymer (SAN)/poly(methyl methacrylate) (PMMA) repeat-use articles intended for contact at room temperature with aqueous, acidic and/or low alcoholic (< 20 %) foodstuffs for less than 1 day and with dry foodstuffs for any duration of contact including long-term storage, is not of a safety concern for the consumer, thus it is updated in this regulation.
What’s more, according to (EU) 2018/79, Table 1 of Annex 1 of (EU) No 10/2011 includes 4 authorized substances. 2,4,4’-trifluorobenzophenone and 2,3,3,4,4,5,5-heptafluoro-1-pentene are authorized as monomer. Tungsten oxide and the mixture of methyl-branched and linear C14-C18 alkanamides, derived from fatty acids, are authorized as an additive or an additive for the production of polymers.
Details of the four new substances are as follows:
FCM Substance No. | CAS No. | Name of Substance | Specific Migration Limit | Restrictions and Norms |
1061 | 80512-44-3 | 2,4,4’-trifluorobenzophenone | Only to be used as a co-monomer in the manufacture of polyether ether ketone plastics up to 0.3 % w/w of the final material. | |
1063 | 1547-26-8 | 2,3,3,4,4,5,5-heptafluoro-1-pentene | Only to be used together with tetrafluoroethylene and/or ethylene co-monomers to manufacture fluorocopolymers for application as polymer processing aid at up to 0.2 % w/w of the food contact material, and when the low-molecular mass fraction below 1 500 Da in the fluorocopolymer does not exceed 30 mg/kg. | |
1064 | 39318-18-8 | tungsten oxide | 0.05mg/kg | Stoichiometry: WOn, n = 2.72-2.90 |
1065 | 85711-28-0 | mixture of methyl-branched and linear C14-C18 alkanamides, derived from fatty acids | 5mg/kg | Only to be used in the manufacture of articles made of polyolefins, and which do not come into contact with foods for which food simulant D2 is assigned in Table 2 of Annex III. |
When tungsten oxide used as reheat agent in polyethylene terephthalate (PET) verification of compliance with the specific migration limit is not required; in all other cases compliance with the specific migration limit shall be verified in accordance with Article 18; the specific migration limit is expressed as mg tungsten/kg food.
Stearamide (FCM substance No 306), which no specific migration limit applies, shall be excluded from verification of the compliance of the migration of the mixture with the specific migration limit laid down for the mixture.
Directive (EU) 2018/79 shall enter into force on the twentieth day following that of its publication in the OJ and be binding in its entirety and directly applicable in all Member States. Prior to the effective date, (EU) 10/2011 compliant plastic materials and products could still be put on the market until February 9th 2019 until they were sold out.
|Further Information:
Notice of the Official Journal of EU
|Our Service:
