Definition of Cosmetics
Under the Cosmetic Supervision and Administration Regulations (CSAR), cosmetics are defined as daily-use chemical products applied to the human body surface such as skin, hair, nails, and lips by smearing, spraying, or other similar methods, for purpose of cleaning, protecting, beautifying, and modifying.
Classification of Cosmetics
Cosmetics can be divided into special cosmetics and ordinary cosmetics:
- Special Cosmetics: Include hair dyeing, hair perming, anti-freckle and whitening, sunscreen, anti-hair loss products, and cosmetics claiming new efficacies.
- Ordinary Cosmetics: Cosmetics that do not fall under the category of special cosmetics, such as products used for general skin/hair care, body care, nail care, hair removal, deodorants, perfumes, makeup, etc. Toothpaste were regulated as ordinary cosmetics as well.
Note: Products intended for hair growth, breast enlargement, or body sculpting are not classified as cosmetics.
Requirements for Cosmetics Enter the Chinese Market
Currently, with the exception of cosmetics sold via cross-border e-commerce channels, which are exempt from registration, other cosmetics (excluding ordinary beauty soaps) must be filed/registered with the National Medical Products Administration (NMPA) or local MPAs prior to their being marketed. China now enforces registration management for special cosmetics and filing management for ordinary cosmetics.
Qualification of Cosmetics Registrant and Filer
Cosmetics registrant or filer is in charge of quality, safety and efficacy claims of cosmetics. And it shall
- Be an enterprise or other organization established according to law;
- Have a quality management system suitable for the cosmetics to be registered and filed; and
- Have an ability of adverse reaction monitoring and evaluation.
Duty of Domestic Responsible Person
Foreign companies have to appoint domestic responsible person to deal with the pre-market registration or filing application. The domestic responsible person shall:
- Register and file the cosmetics or new cosmetic ingredients in the name of the registrant and filer;
- Assist registrants and filers in the monitoring of cosmetics adverse reaction, safety monitoring and reporting of new cosmetic raw materials;
- Assist registrants and filers in the recall of cosmetics and cosmetic ingredients;
- Undertake the corresponding safety and quality responsibilities of cosmetics and new cosmetic ingredients placed in the Chinese market according to the agreement between the responsible person and registrant/filer; and
- Cooperate with the supervision and inspection work of the supervision departments.
Application Process
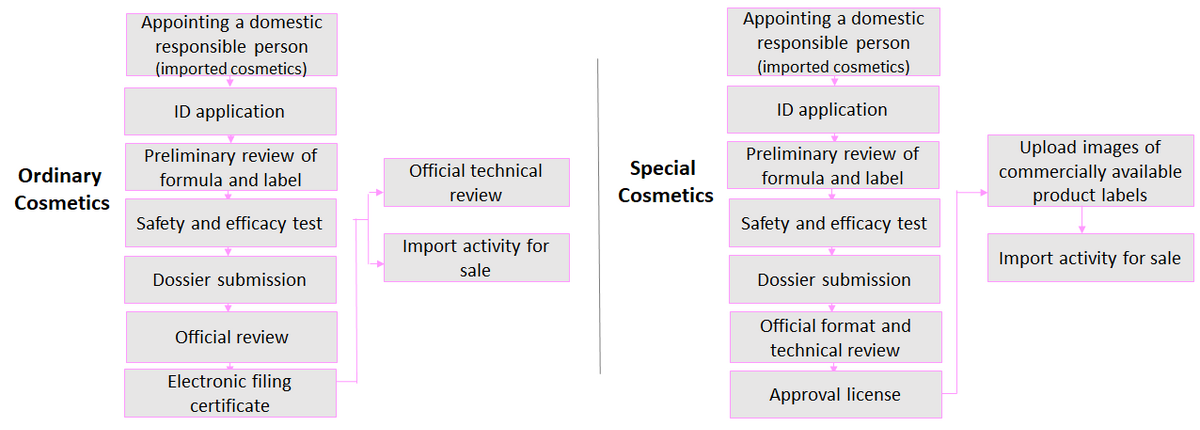
Requirements for Cosmetic Registration and Filing
Materials required for cosmetic registration and filing
According to the Regulations on Cosmetic Registration and Filing Management, the following materials must be submitted for cosmetic registration and filing:
- Cosmetic Registration and Filing Information Form and related documents;
- Product name;
- Product formula;
- Standards implemented by the product;
- Draft of product label;
- Product inspection report; and
- Product safety assessment data.
Testing requirements
The product testing reports used for cosmetic registration and filing must comply with the Cosmetic Safety Technical Specifications, Regulations on Cosmetic Registration and Filing Inspection Work, and other relevant regulations. The tests should cover:
- Microbiological tests;
- Physical and chemical tests;
- Toxicological tests;
- Human safety evaluation (for special cosmetics); and
- Human efficacy evaluation.
Conditions for exemption from toxicological tests:
a) General cosmetic manufacturers must have received certification of their production quality management system from the competent authorities in their country or region.
b) The results of the product safety risk assessment must adequately confirm the safety of the product. If the product is produced by multiple manufacturers, each must have obtained production quality management system certification from the competent authorities in their country or region to qualify for an exemption from submitting toxicological test reports.
Toxicological tests cannot be exempted in the following situations:
- Products claimed to be used by infants and children;
- Products using new cosmetic ingredients still under safety monitoring; and
- Based on the quantitative grading score results, the filer, domestic responsible person, and manufacturers are listed as key regulatory targets.
Cosmetic Labeling Requirements
According to the Cosmetic Label Management Measures, the Chinese label of cosmetics must at least include the following contents:
- Product Chinese name, registration certificate number for special cosmetics;
- Name and address of the registrant or filer, if the registrant or filer is a foreign enterprise, the name and address of the domestic responsible person must also be indicated;
- Name and address of the manufacturer, for domestic cosmetics, the production license number of the manufacturer must also be indicated;
- Standard number implemented by the product;
- Full ingredients;
- Net content;
- Shelf life;
- Usage method;
- Necessary safety warning language; and
- Other content required to be indicated by laws, administrative regulations, and mandatory national standards.
For products with a packaging box, the product Chinese name and shelf life must also be indicated on the packaging container that directly contacts the contents.
Post-Market Supervision
Ordinary Cosmetics
- The competent authorities conduct technical reviews of some filed cosmetics;
- Starting in 2022, filed cosmetics must submit an annual report. Provincial-level and above people's government drug regulatory authorities should organize sampling inspections of cosmetics; for cosmetics with many problems reported or found during routine supervisory inspections, the responsible drug regulatory department can conduct special sampling inspections.
Our Services
| Registration of Special Cosmetics/ Filing of Ordinary Cosmetics |
|
