[AQSIQ] Monthly overview report of AQSIQ notifications on imported goods in August 2017 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China (AQSIQ) validated notifications on 88 batches of imported industrial goods, which were found non-compliant during inspection and quarantine process at the entry ports of China. They have not been offered for sale on the China market yet and are re-dispatched or destroyed instead according to laws. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
I. Analysis on product category and violation
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
II. Analysis of measures taken | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
They have not been offered for sale on the China market yet and are re-dispatched or destroyed instead according to laws. The disposable sanitary products, toys & children’s products, textiles and medical apparatus and instruments not complying with relevant standards are usually destroyed. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
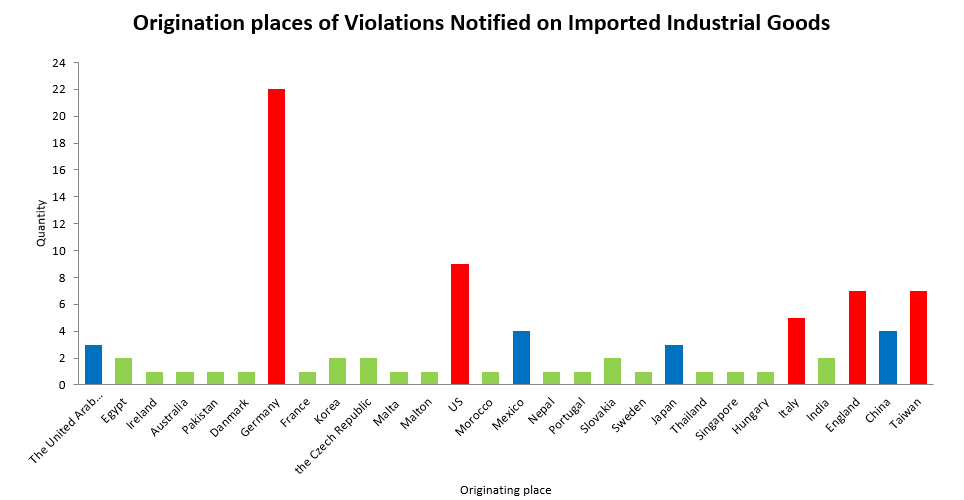
III. Analysis of originating place | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
信息来源:http://jyjgs.aqsiq.gov.cn/jckbhgcp/201704/t20170428_487213.html | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|Our Services China Regulatory Compliance Testing | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
[AQSIQ] Monthly overview report of AQSIQ notifications on imported goods in August 2017